Surfactants: Surface Active Agents
The information on the properties of water previously, provides background for a discussion of the properties of surfactants. A surfactant is briefly defined as a material that can greatly reduce the surface tension of water when used in very low concentrations. Table Two shows that Softanol 90 reduces the surface tension of water from 73 to 30 dynes per centimetre when used at a concentration of 0.005 percent. Ethanol when used at a concentration of 20 percent, however, only reduced tension of water to 38 dynes per centimetre.
Relationship of Surface Tension and Concentration
Table Two
| Percent Concentration required to reduce the surface tension of water to indicated values | |||||
| Surface tension, dynes per cm | 73 | 50 | 40 | 30 | 22 |
| Softanol 90 | 0 | 0.003 | 0.0008 | 0.0050 | --- |
| Ethanol | 0 | 9 | 18 | 40 | 100 |

A particular type of molecular structure performs as a surfactant. This molecule is made up of a water soluble (hydrophilic) and a water insoluble (hydrophobic) component (Figure Two).
Figure Two Schematic Sketch of Surfactant Molecule
The hydrophobe is usually the equivalent of an 8 to 18 carbon hydrocarbon, and can be aliphatic, aromatic, or a mixture of both. The sources of hydrophobes are normally natural fats and oils, petroleum fractions, relatively short synthetic polymers, or relatively high molecular weight synthetic alcohols. The hydrophilic groups give the primary classification to surfactants, and are anionic, cationic and nonionic in nature. The anionic hydrophiles are the carboxylates (soaps), sulphates, sulphonates and phosphates. The cationic hydrophiles are some form of an amine product. The nonionic hydrophiles associate with water at the ether oxygens of a polyethylene glycol chain (Figure Three). In each case, the hydrophilic end of the surfactant is strongly attracted to the water molecules and the force of attraction between the hydrophobe and water is only slight. As a result, the surfactant molecules align themselves at the surface and
polyethylene glycol chain (Figure Three). In each case, the hydrophilic end of the surfactant is strongly attracted to the water molecules and the force of attraction between the hydrophobe and water is only slight. As a result, the surfactant molecules align themselves at the surface and  internally so that the hydrophile end is toward the water and the hydrophobe is squeezed away from the water (Figure Four).
internally so that the hydrophile end is toward the water and the hydrophobe is squeezed away from the water (Figure Four).
Figure Three Polyethylene Glycol Chain
Figure Four Schematic Sketch of Surfactant Molecules in Water
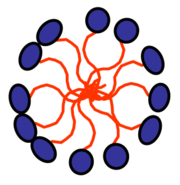
This internal group of surfactant molecules is referred to as a micelle (m).

Because of this characteristic behaviour of surfactants to orient at surfaces and to form micelles, all surfactants perform certain basic functions. However, each surfactant excels in certain functions and has others in which it is deficient.
Foaming agents, emulsifiers, and dispersants are surfactants which suspend respectively, a gas, an immiscible liquid, or a solid in water or some other liquid. Although there is similarity in these functions, in practice the surfactants required to perform these functions differ widely. In emulsification, as an example - the selection of surfactant or surfactant system will depend on the materials to be used and the properties desired in the end product. An emulsion can be either oil droplets suspended in water, an oil in water (O/W) emulsion, water suspended in a continuous oil phase, water in oil (W/O) emulsion, or a mixed emulsion. Selection of surfactants, orders of addition and relative amounts of the two phases determine the class of emulsion.
Each of these three functions is related to the surfactant absorbing at a surface, either gas, liquid or solid with the hydrophilic ends of the molecules oriented to the water phase. The surfactants form what amounts to a protective coating around the suspended material, and these hydrophilic ends associate with the neighbouring water molecules. In addition to surfactant effects the stability of these suspensions is related to the particle size and density of the suspended material.
Solubilisation is a function closely related to emulsification. As the size of the emulsified droplet becomes smaller, a condition is reached where this droplet and the surfactant micelle are the same size.
At this stage, an oil droplet can be imagined as being in solution in the hydrophobic tails of the surfactant and the term solubilisation is used. Emulsions are milky in appearance and solubilised oils, for example - are clear to the eye.
The Function of Detergency
The function of detergency or cleaning is a complex combination of all the previous functions. The surface to be cleaned and the soil to be removed must initially be wet and the soils suspended, solubilised, dissolved or separated in some way so that the soil will not just re-deposit on the surface in question (Figure Five).
The surface to be cleaned and the soil to be removed must initially be wet and the soils suspended, solubilised, dissolved or separated in some way so that the soil will not just re-deposit on the surface in question (Figure Five).
Figure Five Simplified Illustration of Detergency